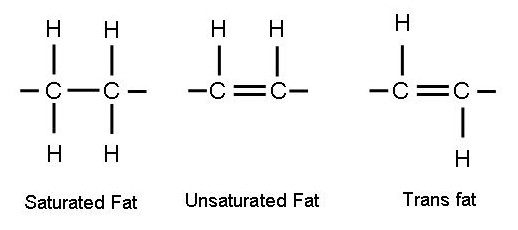
See a lot in the media about "transfats" and I even had myself mentioning them in a conversation with someone not really understanding them.
Can some clarify "transfats" in the context of other fats, our IF way of life and health generally.
What are some good online articles or references you can share?
Can some clarify "transfats" in the context of other fats, our IF way of life and health generally.
What are some good online articles or references you can share?